The water-accessible surface area of an IgG antibody Immunoglobulin G (IgG) is a type of antibody.
IgG immunoglobin represents approximately 75% of serum antibodies in humans.
IgG is the most common type of antibody found in blood circulation.
IgG molecules are created and released by plasma B cells.
Each IgG antibody has two paratopes.
IGG the most abundant antibody type in the bloodstream has a concentration of 10 g/L, second only to albumin with a concentration of 40 g/L: the high concentration of IGG and albumin are due to the plasma half-life of 21 days, exceeding other circulating proteins.
Antibodies are major components of humoral immunity and IgG is the main type of antibody found in blood and extracellular fluid, allowing it to control infection of body tissues.
By binding many kinds of pathogens such as viruses, bacteria, and fungi, IgG protects the body from infection.
IgG-mediated binding of pathogens causes their immobilization and binding together via agglutination.
IgG coating of pathogen surfaces is known as opsonization, and allows their recognitions and ingestion by phagocytic immune cells leading to the elimination of the pathogen itself.
IgG activates the classical pathway of the complement system, a cascade of immune protein production that results in pathogen elimination.
IgG binds and neutralizes toxins.
IgG also plays an important role in antibody-dependent cell-mediated cytotoxicity (ADCC) and intracellular antibody-mediated proteolysis.
IgG is also associated with type II and type III hypersensitivity reactions.
IgG antibodies participate predominantly in the secondary immune response.
IgG is secreted as small in size monomer allowing it to easily diffuse into tissues.
It is the only antibody isotype that has receptors to facilitate passage through the human placenta, thereby providing protection to the fetus in utero.
Residual IgG absorbed through the placenta provides the neonate with humoral immunity before its own immune system develops.
Colostrum contains a high percentage of IgG, especially bovine colostrum.
In individuals with prior immunity to a pathogen, IgG appears about 24–48 hours after antigenic stimulation.
In the first six months of life, the newborn has the same antibodies as the mother and the child can defend itself against all the pathogens that the mother encountered in her life until these antibodies are degraded.
IgG are also involved in the regulation of allergic reactions, including anaphylaxis.
IgG antibodies can prevent IgE mediated anaphylaxis by intercepting a specific antigen before it binds to mast cell–associated IgE.
Consequently, IgG antibodies block systemic anaphylaxis induced by small quantities of antigen but can mediate systemic anaphylaxis induced by larger quantities.
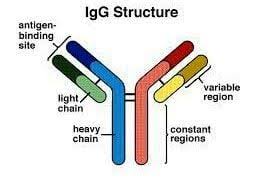
IgG antibodies are large globular proteins made of four peptide chains two identical γ (gamma) heavy chains of about 50 kDa and two identical light chains of about 25 kDa, and has a total molecular weight of about 150 kDa.
The resulting tetramer has two identical halves, which together form a Y-like shape.
The Fc regions of IgGs bear a highly conserved N-glycosylation site at in the constant region of the heavy chain.
The N-glycan composition in IgG has been linked to several autoimmune, infectious and metabolic diseases.
There are four IgG subclasses (IgG1, 2, 3, and 4) in humans, named in order of their abundance in serum (IgG1 being the most abundant).
IgG affinity to Fc receptors on phagocytic cells is specific to individual species from which the antibody comes as well as the class.
Immune response to most antigens includes a mix of all four subclasses, it has been difficult to understand how IgG subclasses can work together to provide protective immunity.
The IgG3, though of relatively low affinity, allows IgG-mediated defenses to join IgM-mediated defenses in clearing foreign antigens.
Subsequently, higher affinity IgG1 and IgG2 are produced.
The balance of these subclasses, in any immune complexes that form, helps determine the strength of the inflammatory processes that follow.
Finally, if antigen persists, high affinity IgG4 is produced, which dampens down inflammation by helping to curtail FcR-mediated processes.
The relative ability of different IgG subclasses to fix complement may explain why some anti-donor antibody responses do harm a graft after organ transplantation.
Adalimumab is an IgG antibody.
IgG antibody levels are generally considered to be indicative of an individual’s immune status to particular pathogens: titers drawn to demonstrate serologic immunity to measles, mumps, and rubella (MMR), hepatitis B virus, and varicella (chickenpox).
Testing of IgG is not indicated for diagnosis of allergy, and there is no evidence that it has any relationship to food intolerances.
