 There are approximately 400,000 cases of infant and childhood hydrocephalus worldwide.
There are approximately 400,000 cases of infant and childhood hydrocephalus worldwide.
Hydrocephalus in early life, if untreated, is detrimental to neural development and can result in death.
Treatment for infant in childhood hydrocephalus requires lifelong monitoring, rapid access to neurosurgical care for patients with acute deterioration and is associated with multiple procedures.
Hydrocephalus implies enlargement of the brain’s ventricles due to a disturbed CSF flow, leading to increased intracranial pressure.
The traditional view that the choroid plexus produces CSF, which flows through the ventricles into the subarachnoid space and is absorbed into the venous system through arachnoid granulations: it is an incompletely understood system and CSF production occurs in the other sites within the cranium, CS flow is multi directional, is influenced by cardiac pulsatility and absorbed unit of CSF occurs in multiple locations.
Congenital or acquired abnormalities can derange CSF circulation and result in hydrocephalus.
Common congenital abnormalities that can cause hydrocephalus are : myelomeningocele, aqueduct stenosis, posterior fossa malformations particularly Chiari and Dandy – Walker malformations.
Acquired disorders commonly associated with hydrocephalus are: tumors, hemorrhage, and infections.
The most common cause of hydrocephalus in the world in infants and children is infection.
Hydrocephalus frequently follows neonatal sepsis due to a variety of pathogens.
In the US the most common cause of hydrocephalus in the pediatric population is associated with intraventricular hemorrhage with prematurity.
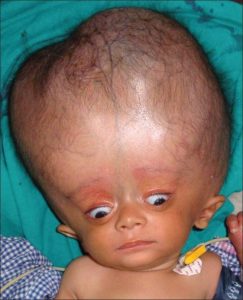
Neonates with hydrocephalus rarely have distress, because of their open fontanelles and skull sutures decompress the additional volume of the expression of the expanded ventricles.
Neonatal hydrocephalus is associated with the larger head circumference than expected, a bulging fontanelle, and widely separated cranial sutures.
The progression of ventricular enlargement may lead to a downward, deviated gaze, irritability, lethargy, episodes of apnea and bradycardia, and developmental regression.
In older children, whose fontanelles have already closed hydrocephalus, has more acute manifestations with headache, nausea, vomiting, double vision, lethargy, and occasionally seizures.
Children with advanced hydrocephalus, will have bradycardia, papilledema, and six nerve palsies, and regression of developmental milestones and difficulties in school.
A CSF shunt is the mainstay of treatment, but has a high failure rate.
The administration of antenatal glucocorticoids and magnesium sulfate to women at risk for preterm delivery has reduced the incidence of germinal matrix hemorrhage.
The use of folic acid supplementation has decreased the incidence myelomeningocele which is often accompanied by hydrocephalus.
Fetal surgery for myelomeningocele is performed in selective patients and has reduced the risk of hydrocephalus by approximately 50%.
Tapping of the lateral ventricle through open fontanelles, or a lumbar puncture can be used to drain CSF, reduce intracranial pressure, and evaluate for infection before implantation of a shunt or temporization device.
The main, long-term alternative to CSF shunting is endoscopic third ventriculostomy, whereby an endoscope is passed through the frontal scalp incision into the third ventricle, and an opening is made in the ventricular floor.
This procedure will allow CSF to flow between the third ventricle and the subarachnoid space below it, and can resolve hydrocephalus symptoms, and reduce ventricular size.
This procedure’s failure rate is less than 5%, but neonate and infants have the highest rates of failure.
The addition of choroid plexus cauterization can increase the success of CSF shunting by endoscopic third ventriculostomy.
Shunting of the lateral ventricles, takes less than an hour to perform, has low risks, but requires general anesthesia.
A simple CSF shunt consists of a proximal ventricular catheter, a valve, and a distal catheter, draining into a body cavity is capable of absorbing the shunted CSF.
Most distal catheters are placed in the peritoneal cavity with extra tubing to allow for the growth of the child and increased distance from the ventricles to the distal end of the catheter.
Alternative sites include the right atrium through the internal jugular or subclavian vein, and the pleural space.
Approximately 30-40% of shunts placed in infants fail in the first year, 40 to 50% fail by two years, and 80% fail by 10 years.
The average patient undergoes, 2.5 shunt revisions during childhood, with 5%, requiring 10 or more revisions.
Shunts fail, primarily for obstruction, that occurs in the ventricular catheter.
It is caused by reactive tissue that occludes the holes in the tubing and is composed predominantly of astrocytes and macrophages.
The second most common cause of failure is it due to bacterial contamination during shunt insertion.
Complications from over drainage can also occur.
