 See Head and cancer IHead and Neck Cancer I
See Head and cancer IHead and Neck Cancer I
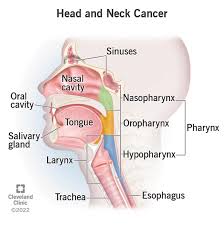
Head and Neck Cancers address tumors arising in the lip, oral cavity, pharynx, larynx, and paranasal sinuses; occult primary cancer, salivary gland cancer, and mucosal melanomas.
It estimated that about 65,630 new cases of oral cavity, pharyngeal, and laryngeal cancers will occur annually, which account for about 3.6% of new cancer cases in the United States.
An estimated 14,500 deaths from head and neck cancers will occur during the same time period.
Squamous cell carcinoma or a variant is the histologic type in more than 90% of these tumors.
Alcohol and tobacco abuse are the most common etiologic factors in cancers of the oral cavity, hypopharynx, larynx, and HPV-unrelated oropharynx.
Patients with H&N cancers are at risk for harboring synchronous primary tumors and developing second primary neoplasms of the H&N, lung, and esophagus.
Presently, HPV associated oropharyngeal cancers are estimated at 60%–70% in the United States.
Stage of H&N cancers at diagnosis predicts survival rates and guides management.
In general, stage I or II disease defines a relatively small primary tumor with no nodal involvement.
Stage III or IV cancers generally include larger primary tumors, which may invade underlying structures and/or spread to regional nodes.
Distant metastases are less common at presentation than in lung and esophagus cancers.
More advanced TNM stages are associated with worse survival.
AJCC Cancer Staging Manual new staging criteria for HPV-related oropharyngeal cancer: nodal disease could be considered stage I if the nodes are ipsilateral and none larger than 6 cm.
Single-modality treatment with surgery or RT is generally recommended for the approximately 30%–40% of patients who present with early-stage disease (stage I or II).
Surgery and RT result in similar survival for many H&N cancers.
Surgery is usually preferred for oral cavity and paranasal sinus cancers, while RT with or without chemotherapy is nearly always preferred for all stages of nasopharyngeal carcinoma.
Combined modality therapy is generally recommended for the approximately 60% of patients with locally or regionally advanced disease at diagnosis.
When chemotherapy is used with radiation, cisplatin is the preferred radiosensitizing agent.
Managing and preventing sequelae after surgery, RT, and systemic therapy is a major concern.
Adequate nutritional support is vital in patients receiving treatment of H&N cancers.
Dental care to prevent and treat RT effects is required.
Evaluation by a speech-language/swallowing therapist before and after treatment is recommended.
Patients are at risk for depression from H&N cancer and its sequelae.
Fertility/reproductive counseling should be offered to younger patients.
Patients should be encouraged to stop smoking, and remain abstinent.
Patients should modify alcohol consumption, if excessive, because these habits decrease the efficacy of treatment and adversely affect other health outcomes.
Typically, unresectable tumors involve the cervical vertebrae, brachial plexus, deep muscles of the neck, or carotid artery.
Tumor involvement of certain sites is associated with poor prognosis: direct extension to involve the external skin; mediastinal structures, prevertebral fascia, or cervical vertebrae.
Patients with resectable tumors who can also be adequately treated without surgery: Definitive treatment with RT alone or RT combined with systemic therapy may represent equivalent or preferable approaches to surgery in some individuals.
Comorbidity is known to be a strong independent predictor for mortality in patients with H&N cancers.
Initial imaging of the primary site is done with CT and/or MRI.
MRI is preferred over CT in patients with cranial nerve symptoms or to evaluate cranial nerve involvement or tumors that encroach on the skull base.
CT, conversely, is complementary to MRI for evaluation of bony erosion or cartilage invasion that may occur with some H&N tumors.
FDG-PET/CT is superior for detecting locoregional nodal and distant metastases in patients with H&N cancers.
There is a sensitivity and specificity value of 91% and 87%, respectively, for detection of regional nodal metastasis by FDG-PET/CT compared with 63% for CT and/or MRI.
PET/CT had a sensitivity value of 89% and a specificity value of 98% for detecting bone metastases in patients with H&N cancer.
With oral, pharyngeal, or laryngeal cancer, FDG-PET/CT detected distant metastasis more often than chest X-ray/H&N MRI and chest CT/H&N MRI.
H&N cancers rarely metastasize to the brain by a hematogenous route.
For patients with locoregionally advanced disease who have undergone surgery, postoperative imaging is recommended if there are signs of early recurrence, or for patients considered at high risk for early recurrence.
Patients with positive margins, advanced T or N stage, or oral cavity cancers are at particular risk for rapid recurrence after surgery.
These patients may also continue to be observed if the clinical examination is reassuring.
Recurrences in patients with head and neck cancer tend to occur in the first 3 years after treatment.
There is little evidence to support imaging surveillance in the long-term more than 6 months after treatment in patients who have negative imaging.
Delayed or late recurrences are more common in patients with HPV-related H&N cancer.
FDG PET/CT showed high sensitivity (92%) and specificity (91%) for detection of H&N cancer recurrence.
H&N cancer treatment can result in fibrosis and altered anatomy, which frequently leads to challenges finding recurrent disease.
Surveillance should take into account tumor site, stage, prognostic factors, presence of symptoms, and changes based on clinical exam.
Neck ultrasound may be used to evaluate suspected nodal disease.
Clinical examination, routine annual imaging using the pretreatment imaging modality (usually CT or MRI) may be indicated.
Nutritional management and supportive care for patients with H&N cancers are indicated, as they are prone to dehydration and weight loss, which can often be severe, as a result of treatment-related toxicity, disease, and health behaviors such as poor nutritional habits.
A speech-language/swallowing therapist should be used throughout the continuum of care.
Long-term swallowing and dental dysfunction are particular risks that are worsened by multimodality therapy and require long-term specialized attention.
Oral mucositis, or tissue damage, is common in patients treated with RT for H&N cancers, though use of intensity-modulated RT [IMRT] may decrease the incidence and duration of this damage.
Oral mucositis causes pain in the mouth and swallowing, affecting the ability to eat and drink, and is associated with breaks and/or delays in treatment, as well as hospitalization.
Oral mucositis is worse in patients receiving concurrent systemic therapy/RT.
Reduction in mucositis pain during the first 4 hours of treatment is significantly greater in the patients who receive diphenhydramine-lidocaine-antacid mouthwash or the doxepin mouthwash compared with placebo.
Treatment with gabapentin for pain from oral mucositis is associated with a reduced need for narcotic pain medication and high doses of opioids.
Very high-dose prophylactic gabapentin (2,700 mg daily) also reduces the number of patients requiring narcotics for oral mucositis.
It is recommended that consideration of doxepin, diphenhydramine-lidocaine-antacid mouthwash, or gabapentin for pain related to oral mucositis.
Feeding tube placement is appropriate in selected patients with H&N cancers.
Advantages of prophylactic tube placement include reductions in hospitalizations, weight loss, and improved quality of life.
Tube feedings are also associated with disadvantages, such as longer dependence on feeding tubes and worse long-term functional outcomes, compared with a reactive approach.
Recommendations for prophylactic tube placement: high-risk patients with severe pretreatment weight loss, ongoing dehydration or dysphagia, significant comorbidities, severe aspiration, anticipated swallowing issues.
Efforts must be made with the help of speech and language pathologists to ensure that patients continue to swallow to prevent severe fibrosis and permanent feeding tube dependence.
With swallowing therapy, adequate pain control, and access to intravenous fluids, feeding tubes can be avoided in most treate head and neck cancer patients.
Prophylactic tube placement is not recommended in lower-risk patients.
Patients with H&N cancers are at risk for oral and dental complications after surgery or RT because of treatment-induced xerostomia and salivary gland dysfunction, which are associated with increased dental caries.
RT to the salivary and oral soft tissues is also associated with bone demineralization and trismus of the masticatory muscles.
Using IMRT and limiting the RT dose to the salivary glands and oral cavity have been shown to decrease xerostomia and damage to the teeth.
Dental/oral evaluation and management can help decrease dental caries and associated problems such as dentoalveolar infection and osteoradionecrosis.
A dental/oral treatment plan needs to be implemented before RT:1) eliminating potential sources of infection; (2) if performing dental extractions, allow adequate time for healing before RT; (3) treating active dental caries and periodontal disease; (4) treating oral candidiasis; and (5) educating patients about preventive strategies.
To decrease oral and dental complications: decreaing dry mouth (eg, by using salivary substitutes and stimulation); (2) reduce risk of dental caries (eg, by using topical fluoride);(3) decrease dentoalveolar infection;(4) prevent and address osteoradionecrosis;(5) decrease trismus of the masticatory muscles and; (6) have patient undergo evaluations during and after treatment to help minimize complications.
Very advanced H&N cancers include (1) newly diagnosed locally advanced T4b (M0); (2) newly diagnosed unresectable regional nodal disease, typically N3; (3) metastatic disease at initial presentation (M1); or (4) recurrent or persistent disease.
The treatment goal is usually cure for patients with newly diagnosed locoregional but unresectable disease.
For patients with recurrent disease, the goal is cure if surgery or radiation remains feasible, or palliation if the patient has received previous RT and the disease is unresectable.
For patients with widely metastatic disease, the goal is palliation or prolongation of life.
The treatment of patients with unresectable locoregional, persistent, recurrent, or metastatic H&N cancers is dictated by the patient’s performance status (PS) and intent of treatment (ie, palliative vs curative).
For newly diagnosed locoregionally advanced disease trials and meta-analyses. show significantly improved overall survival (OS), disease-free survival, and locoregional control when a systemic therapy and radiation regimen is compared with RT alone for locoregionally advanced disease: cisplatin plus RT.
Epidermal growth factor receptor (EGFR) overexpression is common in squamous cell H&N cancers and is associated with poor survival outcomes: RT with cetuximab significantly improves overall compared with RT alone.
The addition of cetuximab to cisplatin and RT did not significantly improve OS in patients with stage III or IV H&N cancer and, importantly, was more toxic.
Cetuximab and RT was compared with cisplatin and RT in 2 randomized phase III trials as a deintensification treatment strategy for HPV-associated locally advanced oropharyngeal cancer, but proved inferior to cisplatin in this setting in terms of OS and was also not better tolerated.
In the RTOG 1016 patients with locally advanced HPV-positive oropharyngeal cancer were randomized to receive accelerated IMRT with either cetuximab or cisplatin.
After a median follow-up of 4.5 years, the cetuximab arm did not meet the criterion for noninferiority.
Five-year OS was 77.9% for the cetuximab arm and 84.6% for the cisplatin arm.
PFS and risk of locoregional failure were significantly worse in the cetuximab arm compared with the cisplatin arm with 5-year PFS and locoregional failure rates being 67.3% and 17.3% for the cetuximab arm, and 78.4% and 9.9% for the cisplatin arm, respectively.
Studies demonstrate that cetuximab and RT is inferior to cisplatin and RT in patients with HPV-related oropharyngeal cancer.
For patients with metastatic disease at initial presentation, palliative adjunctive measures include analgesics and other measures to control manifestations of disease spread.
Locoregional treatment (eg, surgery, RT, or ablative therapies) may be used for oligometastatic disease.
Response rates to single-agent therapies range from 15% to 35%.
Randomized trials assessing a cisplatin-based combination regimen (cisplatin/5-FU) versus single-agent therapy with cisplatin, 5-FU, or methotrexate showed significantly higher response rates, but no difference in OS and greater toxicity for the combination regimen.
Complete response is associated with longer survival and, although infrequent, has been reported more often with combination regimens.
A phase III randomized trial (EXTREME) of 442 patients found that cetuximab plus cisplatin/5-FU or carboplatin/5-FU improved median survival compared with the standard chemotherapy doublet of platinum/5-FU (10.1 vs 7.4 months.
The response rate was improved with the addition of cetuximab (36% vs 20%.
A randomized phase III trial found no significant difference in survival when comparing cisplatin/5-FU and cisplatin/paclitaxel.
Trials evaluating immune checkpoint inhibitors demonstrated efficacy in patients with recurrent or metastatic HNSCC.
Pembrolizumab, an anti-PD-1 antibody, was evaluated as a first-line option for recurrent or metastatic HNSCC in the KEYNOTE-048 trial: Patients randomized to receive pembrolizumab, pembrolizumab with a platinum and 5-FU: OS benefit was observed in the pembrolizumab with a platinum and 5-FU arm, compared with the EXTREME arm (median OS, 13 vs 10.7 months, respectively.
Based on the results of KEYNOTE-048 pembrolizumab/platinum/5-FU a preferred first-line option for all patients with recurrent, unresectable, or metastatic disease who have no surgical or radiotherapeutic option.
Other combination regimens recommended by the panel for treatment of metastatic HNSCC include (1) cisplatin or carboplatin, plus 5-FU with cetuximab; cisplatin or carboplatin, plus a taxane; cisplatin with cetuximab; cisplatin with 5FU; or cetuximab with a platinum and a taxane.
Single agents recommended by the panel include cisplatin, carboplatin, paclitaxel, docetaxel, 5-FU, methotrexate, capecitabine, and cetuximab.
Surgery is recommended for resectable recurrent or persistent locoregional disease, in the absence of distant metastatic disease.
Patients with resectable recurrent or persistent locoregional disease who have not previously been treated with RT may also be treated with concurrent systemic therapy/RT.
If the recurrence is unresectable and the patient had not had prior RT, then RT with concurrent systemic therapy is recommended.
For patients with recurrent disease who are not amenable to curative-intent radiation or surgery, the treatment approach is the same as that for patients with metastatic disease.
Nivolumab in a phase III randomized clinical trial including 361 patients with recurrent HNSCC whose disease had progressed within 6 months after platinum-based chemotherapy: median follow-up of 5.1 the OS was significantly greater in patients given nivolumab compared with patients given standard second-line single-agent systemic therapy (methotrexate, docetaxel, or cetuximab.
OS benefit in patients treated with nivolumab appeared to be confined to those patients with a tumor PD-L1 expression level of 1% or more, 8.7 vs 4.6 months; HR.
In patients with tumor PD-L1 expression level <1%, no OS advantage was shown for the nivolumab-treated patients.
These results indicate that nivolumab prolongs survival in patients with recurrent or metastatic HNSCC cancer that has progressed after platinum-based chemotherapy, relative to patients who receive standard single-agent systemic therapy.
Pembrolizumab showed a 1-year OS rate of 38%.
Among the patients who showed a response85% of the responses lasted 6 months or longer, and 71% lasted 12 months or longer.
The results for OS were significantly better with pembrolizumab only for patients with tumors that have PD-L1 expression.
The nonrandomized phase II KEYNOTE-055 trial studied pembrolizumab in patients with HNSCC that progressed after treatment with both a platinum and cetuximab.
The overall response rate was 16%, and the mean duration of response was 8 months.
Afatinib was compared with methotrexate in patients with recurrent or metastatic H&N cancer who had progressed on or after platinum-based therapy.
Patients randomized to receive afatinib had greater PFS compared with patients randomized to receive methotrexate (2.6 vs 1.7 months, with no significant differences for OS.
The panel recommends immunotherapy withnivolumab and pembrolizumab is a category 1 preferred option for patients with recurrent or metastatic HNSCC who have progressed on or after platinum-based chemotherapy based on high-quality evidence.
PD-L1 expression may be associated with better outcomes from treatment with immunotherapy for recurrent or metastatic HNSCC.
Afatinib has a PFS benefit, but not an OS benefit, over methotrexate and is a category 2 systemic therapy option for non-nasopharyngeal persistent H&N cancer or cancer that has progressed on or after platinum-containing chemotherapy.
Salivary gland tumors can arise in the major salivary glands (parotid, submandibular, sublingual) or in one of the minor salivary glands, which are widely spread throughout the aerodigestive tract.
Many minor salivary gland tumors are located on the hard palate.
Approximately 20% of the parotid gland tumors are malignant.
The incidence of malignancy in submandibular and minor salivary gland tumors is approximately 50% and 80%, respectively.
These malignant tumors constitute a broad spectrum of histologic types, including mucoepidermoid, acinic, adenocarcinoma, adenoid cystic carcinoma, malignant myoepithelial tumors, and squamous cell carcinoma.
The primary diagnosis of squamous cell carcinoma of the parotid gland is rare; however, the parotid gland is a frequent site of metastasis from skin cancer.
Prognosis and tendency to metastasize vary among these histologic types.
Major prognostic factors are histologic grade, tumor size, and local invasion.
Staging is done using the AJCC Cancer Staging Manual.
The major therapeutic approach for salivary gland tumors is adequate and appropriate surgical resection.
Surgical intervention requires careful planning and execution, particularly in parotid tumor surgery, because the facial nerve is in the gland.
The gland should be preserved if the nerve is not directly involved by the tumor.
Most parotid gland tumors are located in the superficial lobe.
If the facial nerve is functioning preoperatively, the nerve can be preserved in most patients.
The facial nerve should be sacrificed if there is preoperative facial nerve involvement with facial palsy or if there is direct invasion of the tumor into the nerve where the tumor cannot be separated from the nerve.
Malignant deep lobe parotid tumors are quite rare.
Highly conformal RT techniques such as IMRT, proton, or other heavy ions is recommended for definitive radiation treatment of salivary gland tumors.
Results from a retrospective cohort study of patients with salivary gland tumors showed better local control and survival outcomes with neutron therapy, relative to photon therapy.
The risk of late effects with neutron therapy is high and tends to increase over time, with estimates as high as 20% at 9 years.
Neutron therapy is no longer recommended for salivary gland cancers.
Most malignant deep lobe parotid tumors will require postoperative RT due adverse features such as the limitations of surgical margins.
RT is also used adjuvantly for tumors with other adverse features:high grade, T3–4 tumors, or positive lymph nodes/
Efficacy data for systemic therapy/RT for patients with advanced salivary gland tumors that have been resected are limited.
Systemic therapy may be used for palliation in advanced disease .
Patients with tumors that are androgen receptor–positive receive androgen receptor therapy (eg, leuprolide, bicalutamide).
Patients with advanced NTRK gene fusion-positive cancer (with 22%–38% being salivary gland tumors) showed objective response rates of 75%–100% with the tyrosine receptor kinase (TRK) inhibitor larotrectinib.
The FDA recently approved larotrectinib and entrectinib for treatment of patients with NTRK gene fusion-positive tumors, and the panel also recommends NTlarotrectinib and entrectinib for patients with recurrent NTRK gene fusion-positive salivary gland tumors and distant metastases.
Finally, HER2 positivity has also been found in some advanced salivary gland tumors, and should receive a HER2-targeted treatment option such as trastuzumab.
Small series demonstrate that ado-trastuzumab emtansine may be active in patients with previously treated metastatic HER2-positive salivary gland cancers.
Various combinations of chemotherapy with agents cisplatin/cyclophosphamide/doxorubicin and cisplatin/vinorelbine have been shown to be active for some salivary gland malignant histologies, with overall response rates ranging from 27% to 60%.
A phase II trial including 32 patients with recurrent or metastatic adenoid cystic carcinoma showed a disease control rate of 88% (partial response of 15.6%, stable disease in 75%) for lenvatinib.
For recurrent or metastatic adenoid cystic carcinoma, lenvatinib is a category option.
Use of other tyrosine kinase inhibitors such as axitinib, sorafenib sunitinib, and dovitinib have been evaluated in phase II trials for salivary gland tumors.
