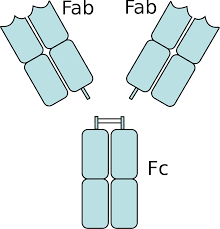
 The fragment crystallizable region (Fc region) is the tail region of an antibody that interacts with cell surface receptors called Fc receptors and some proteins of the complement system.
The fragment crystallizable region (Fc region) is the tail region of an antibody that interacts with cell surface receptors called Fc receptors and some proteins of the complement system.
The Fc region allows antibodies to activate the immune system, through binding to Fc receptors.
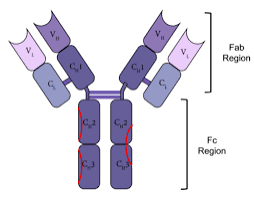
In IgG, IgA and IgD antibody isotypes, the Fc region is composed of two identical protein fragments, derived from the second and third constant domains of the antibody’s two heavy chains.
In IgM and IgE Fc regions contain three heavy chain constant domains in each polypeptide chain.
Glycosylation of the Fc fragment is essential for Fc receptor-mediated activity.
An antibody digested by papain yields three fragments, two Fab fragments and one Fc fragment
An antibody digested by pepsin yields two fragments: a F(ab’)2 fragment and a pFc’ fragment
The Fab region, contains variable sections that define the specific target that the antibody can bind.
The Fc region of all antibodies in a class are the same for each species; they are constant rather than variable.
Fc binds to various cell receptors and complement proteins.
The Fc receptor protects IgG from degradation.
In this way, it mediates different physiological effects of antibodies (opsonization, cell lysis; degranulation of mast cells, basophils, and eosinophils; and other processes).
The Fc region of immunoglobulins has been engineered to contain an antigen-binding site called Fcab.
Fcab fragments can be inserted into a full immunoglobulin by swapping the Fc region, thus obtaining a bispecific antibody, containing a both Fab and Fcab regions containing distinct binding sites.
These are bispecific monoclonal antibodies.
