In patients with coronary artery disease the degree of luminal narrowing, plaque burden and characteristics, and physiological significance are of prognostic importance.
Coronary angiography is the standard method to evaluate CAD in guiding percutaneous coronary intervention, but other measurements are used for incremental information.
Intravascular ultrasound can provide detailed anatomical information regarding the lumen, vessel, and plaque in coronary arteries.
Intravascular ultrasound can guide the PCI procedure to improve stent placement and minimize stent related problems has been reported to improve outcomes in comparison with angiography guided PCI.
Fractional flow reserve is invasive physiologic index that helps determine whether the stenosis is causing ischemia and is associated with fewer clinical events than angiography guided PCI and medical treatment.
For patients with coronary artery disease being evaluating for PCI, procedures can be guided by fractional flow reserve or intravascular ultrasonography for decision making regarding revascularization and stent implantation: these two techniques with respect to outcome of death, myocardial infarction, or revascularization at 24 months were not inferior.
Among patients with complex coronary artery lesions, intravascular imaging by PCI has a lower risk of death from cardiac causes, target vessel, related myocardial infarction, or clinically driven target vessel revascularization than angiographic guided PCI (Lee JM).
Patients with complex coronary artery lesions who undergo PCI have worse clinical outcomes, than patients who undergo PCI for coronary artery lesions that are not complex.
Bare-metal stents provide an increased arterial lumen diameter immediately post procedure.
Today one in four PCI patients in the United States is over age 75,
The need for repeat vessel revascularization in less complex lesions is 10-15% and 2-3 times higher in more complex lesions when bare-metal stents are used.
Restenosis occurs primarily as the result of neointimal hyperplasia.
Bare metal stents minimize the risk of infarct vessel reocclusion and reinfarction compared with balloon angioplasty, but are associated with restenosis due to neointimal hyperplasia.
First generation drug eluting stents that release sirolimus or paclitaxel reduce risk of of restenosis after PCI, compared to bare metal stents: bit such stents are associated with late stent thrombosis, which may be associated with nonfatal myocardial infarction or death from cardiac causes.
First generation drugs eluting stents had stainless steel platforms.
All metallic stents remain susceptible to late stent thrombosis and restenosis and
studies suggest adverse events originating from the target lesion occur at 2 to 3% per
year for at least five years with no plateau evident.
New generation stents release everilomus or zotarolimus and have cobalt-chrome or platinum-chrome platforms with thinner strut thickness and more biocompatible, durable polymer coating.
New generation drug eluting stents have almost completely replaced paclitaxel eluting stents and sirolimus eluting stents are no longer made.
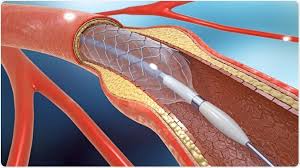
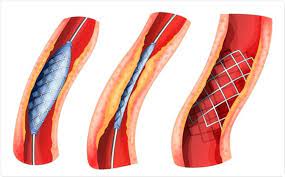
Stents were developed that would maintain vessel patency following angioplasty.
All stents consist of an underlying scaffold.
Most stents are balloon-expandable.
Early stents primarily used stainless steel, while newer stents use cobalt-chromium and platinum-chromium alloys, with greater radial strength.
Newer materials allow the stent struts to become thinner at approximately 75 µm.
Stents with thin struts have lower rates of restenosis than those with thick struts.
Stents promote restenosis by causing proliferation of vascular smooth-muscle cells.
To reduce this neointimal hyperplasia, drugs with antiproliferative effects are coated onto the metal scaffolds:drug-eluting stents (DES).
Superiority of DES over bare-metal stents (BMS) has been demonstrated with the need for repeated revascularization reduced by 50% to 70%.
A DES includes a polymer layer in addition to the metallic strut.
The polymer is the vehicle for and ensures a controlled release of the antiproliferative drugs.
The two most clinically important classes of antiproliferative agents are paclitaxel and sirolimus analogues.
Drug elution is relatively rapid, with 100% of zotarolimus released within 30 days.
Polymers are crucial in effective drug delivery, but their persistence may cause inflammatory responses, leading to thrombosis, and stenosis.
DES with biodegradable polymers and even novel polymer-free DES are available to target lesion failure at 12 months.
Bioresorbable Drug-Eluting Stents
Despite DES technology, late stent thrombosis and target lesion revascularization remain as the
presence of metallic struts can promote in-stent neoatherosclerosis, recurrent stent thrombosis, and restenosis.
Drug eluting stents should inhibit neo intimal hyperplasia and permit physiological arterial healing with smooth endothelial coverage of all stent struts.
Drug eluting stents may be associated with excessive anti-proliferative effects, persistence of stent components, including polymer coatings, leading to chronic inflammation, impaired arterial healing, and risk of thrombotic events.
Thrombosis in a stent is associated with poor clinical outcomes, with one in five patients dying and more than 2/3 have a significant myocardial infarction.
Premature discontinuance of antiplatelet therapy is one of the most important predictors of stent thrombosis.
Increased risk of the stent thrombosis with malapposition of stent, delayed endothelialization, and hypersensitivity reactions.
Causes re-stenosis when treated plaque fractures during the process of percutaneous coronary angioplasty and platelets, inflammatory cells, monocytes, and neutrophils migrate to the site of injury and release cytokines and growth factors which cause medial vascular smooth muscle proliferation which provides an extracellular matrix resulting in neointimal hyperplasia.
After coronary artery stent placement most of the lumen loss is clinically evident within 6-9 months.
Antiproliferative agents sirolimus and paclitaxel coupled with polymers elute slowly to inhibit neointimal tissue growth.
Patients receiving sirolimus eluting stents have a lower risk of restenosis compared to paclitaxel eluting stents.
Zotarolimus eluting stent with a phosphorycholine durable polymer is a second generation drug eluting stent with safety and efficacy.
With zotarolimus stents patients with stable coronary artery disease or low risk acute coronary syndrome treated with three months of dual antiplatelet therapy was not inferior to 12 months of therapy, without significantly increasing the risk of stent thrombosis (OPTIMIZE Triall Investigators).
Incidence of early blood vessel closure after coronary stent placement markedly reduced with use of thienopyridine agents and with the addition of aspirin risk of subacute thrombosis after bare metal stent placement is 0.5-1.9%.
Recommended that following procedure antiplatelet regimen of aspirin and clopidogrel be utilized unless contraindicated.
Diabetics have poorer results than nondiabetics but bringing HbA1C to less than 7% yields equivalent results.
Patients having noncardiac with coronary artery stents have increased risk of CV events in postop period.
After stent placement noncardiac is associated with incidence of death or major adverse cardiac events of 2-9%, and the risk is higher for patients early after implantation.
Stent placement associated with increased CV risks due to underlying atherosclerosis, prothrombotic , and proinflammatory surgical manifestations and risk of stent thrombosis.
Stent thrombosis risks are increased by stent malapposition, delayed endothelialization, and hypersensitivity reactions.
Peri operative cardiac risk in patients with coronary artery stents undergoing noncardiac is greatest in the first 4-6 weeks following stent placement, and diminishes over time, irrespective of the type of stent placed.
Noncardiac surgery should be postponed for 4-6 weeks following placement of a bare metal coronary stent and 6-12 months following the implantation of the drug eluting stent.
Avoiding surgery for 12 months after placement of a drug eluting stent is currently recommended.
MIs more commonly originate in the LAD, left circumflex, and right coronary arteries and that over half of these MIs originate in the proximal portion of these vessels.
The hot spots for prophylactic stenting would thus be within the first 20 mm of both the left anterior descending and left circumflex arteries and between 15 mm and 40 mm in the right coronary artery.
Approximately 5% of patients undergo noncardiac surgery in the one year following coronary stent placement.
An estimated one in eight coronary stents used with nonacute indications (U.S.).
Patients with coronary artery stents that undergo noncardiac surgery have increased risk of adverse cardiovascular events
Perioperative stent thrombosis results from incomplete stent epithelization, discontinuance of antiplatelet therapy, and pro thrombotic state caused by surgery and is associated with significant morbidity and mortality.
The risk of perioperative stent thrombosis is estimated at 0.86% to 2.33% and is highest early after stent implantation and declines over time (Alshawabkleh LI et al).
Surgery is generally considered safe is performed more than six weeks after bare metal stent implantation, but for stents that are drug eluting the risk remains for thrombosis, even years after because of delayed stent endotheliazation due to the drug.
Unfractionated or low molecular weight heparin being substituted for dual antiplatelet therapy in the period does not prevent stent thrombosis and may increase bleeding rates.
The overall incidence of death or major adverse cardiac events is 2-9% following stent placement and noncardiac , and is higher when is performed early after stent implantation.
The increased risk of adverse cardiovascular events in patients with stents relates to the underlying atherosclerotic disease, the prothrombotic and inflammatory effects of , and the risk stent thrombosis.
Discontinuing antiplatelet therapy for dental or minor surgical procedures is not advised.
Continuation of at least one antiplatelet agents or preferably two antiplatelet agents decreases the risk for perioperative stent thrombosis, if the effects on perioperative bleeding risk are considered acceptable.
Blood loss is increased by approximately 20% with aspirin therapy alone and approximately 50% with dual antiplatelet therapy for stents, without affecting mortality: In most cases can be performed safely with the patient receiving dual antiplatelet therapy or aspirin alone.
In surgical procedures which require the premature discontinuation of antiplatelet therapy in patients with stents to decrease bleeding risk, the agents should be stopped 5-7 days prior to noncardiac and restarted immediately following at the earliest opportunity.
Bioresorbable vascular scaffolds as stents do not lead to different rates of device
oriented adverse events at one year follow-up compared to everolimus metallic stance eluting stents (Sone GW et al).
Percutaneous coronary intervention using a contemporary drug-eluting stent on a shortened regimen of dual antiplatelet therapy proved significantly more effective and equally safe as a bare metal stent in elderly patients in a randomized, multicenter SENIOR trial.
The shortened time for dual platelet therapy minimizes the risk of bleeding complications, which is typically high in the elderly.
Modern drug eluting stents in combination with the shortened 1- or 6-month dual antiplatelet therapy duration results in fewer major adverse cardiac and cerebrovascular events with no increase in bleeding, compared with bare metal stents.
The SENIOR was a single-blind, randomized trial of 1,200 PCI patients age 75 and older at 44 centers: 45% of participants had an acute coronary syndrome, 55% had stable or silent CAD.
If they had stable CAD, they were slated for 1 month of dual antiplatelet therapy (DAPT), and if they had acute coronary artery syndrome, they got 6 months of DAPT.
in the above study the primary composite efficacy endpoint was the 1-year rate of all-cause mortality, acute MI, stroke, or ischemia-driven target lesion revascularization. The rate was 11.6% in the drg-eluted stent (DES) group and 16.4% in the bare metal stent group, for a 29% reduction in favor of the DES strategy.
Among patients at high bleeding risk who received dual antiplatelet therapy after percutaneous intervention, the use of polymer based eluding stent was non-inferior to the use of polymer-free drug coated stents with regard to safety and effectiveness outcomes.
Among patients with severe ischemic left ventricular systolic dysfunction who receive optimal medical therapy, PCI revascularization does not result in a lower incidence of death from any cause or hospitalization for heart failure.
In the above trial patients with severe left ventricular systolic dysfunction, extensive coronary disease, and dysfunctional but viable myocardium who received optimal medical therapy, addition of revascularization by PCI did not result in lower incidence of death from any cause or hospitalization for heart failure, incremental improvement in left ventricular ejection fraction, or a sustained difference in quality of life and a median of 3.4 years.
Among high-risk patients who had undergone PCI, follow up strategy of routine functional stress testing, as compared with standard care alone, does not improve clinical outcomes at two years (Duk-Woo Park).
