 Cardiac muscle is one of three types of muscle tissues, with the other two being skeletal muscle and smooth muscle.
Cardiac muscle is one of three types of muscle tissues, with the other two being skeletal muscle and smooth muscle.
It is an involuntary, striated muscle that constitutes the main tissue of the wall of the heart.
The cardiac muscle or myocardium forms a thick middle layer between the outer layer of the heart wall, the pericardium, and the inner layer, the endocardium, with blood supplied via the coronary circulation.
Cardiac muscle is composed of individual cardiac muscle cells joined by intercalated discs, and encased by collagen fibers and other substances that form the extracellular matrix.
It contracts in a similar manner to skeletal muscle, although with some important differences.
Electrical stimulation in the form of a cardiac action potential triggers the release of calcium from the cell’s internal calcium store, the sarcoplasmic reticulum.
The rise in calcium causes the cell’s myofilaments to slide past each other in a process called excitation-contraction coupling.
Diseases of the heart muscle known as cardiomyopathies include ischemic conditions caused by a restricted blood supply to the muscle such as angina, and myocardial infarction.
Cardiac muscle tissue or myocardium forms the bulk of the heart.
The heart wall is a three-layered structure with myocardium sandwiched between the inner endocardium and the outer epicardium.
The endocardium covers the cardiac valves, and joins with the endothelium that lines the blood vessels that connect to the heart.
The outer aspect of the myocardium, the epicardium forms part of the pericardial sac that surrounds, protects, and lubricates the heart.
The myocardium has several sheets of cardiac muscle cells.
The sheets of muscle of the left ventricle closest to the endocardium are oriented perpendicularly to those closest to the epicardium.
These sheets contract in a coordinated manner squeezing the left ventricle in several directions simultaneously: longitudinally, radially and with a twisting motion squeezing the maximum possible amount of blood out of the heart with each heartbeat.
The contracting heart muscle requires a constant flow of blood to provide oxygen and nutrients, brought to the myocardium by the coronary arteries.
Coronary arteries originate from the aortic root and lie on the outer or epicardial surface of the heart.
Blood is then drained away by the coronary veins into the right atrium.
Cardiac muscle cells, the cardiomyocytes, are the contractile myocytes of the cardiac muscle.
The cells are surrounded by an extracellular matrix produced by supporting fibroblast cells.
Specialized modified cardiomyocytes known as pacemaker cells, set the rhythm of the heart contractions.
The pacemaker cells are weakly contractile without sarcomeres, and are connected to neighboring contractile cells via gap junctions.
Pacemaker cells are located in the sinoatrial node, which is the primary pacemaker, positioned on the wall of the right atrium, near the entrance of the superior vena cava.
Other pacemaker cells are found in the atrioventricular node, the secondary pacemaker.
Pacemaker cells carry the impulses that are responsible for the beating of the heart.
Pacemaker cells are distributed throughout the heart and are responsible for being able to spontaneously generate and send out electrical impulses.
They also must be able to receive and respond to electrical impulses from the brain.
They must be able to transfer electrical impulses from cell to cell.
Pacemaker cells in the sinoatrial and atrioventricular nodes are smaller and conduct at a relatively slow rate between the cells.
Specialized conductive cells in the bundle of His, and the Purkinje fibers are larger in diameter and conduct signals at a fast rate.
The Purkinje fibers rapidly conduct electrical signals; coronary arteries to bring nutrients to the muscle cells, and veins and a capillary network to take away waste products.
Cardiac muscle cells are the contracting cells that allow the heart to pump.
Each cardiomyocyte needs to contract in coordination with its neighboring cells as a functional syncytium.
If this coordination breaks down then – the heart may not pump at all, such as may occur during abnormal heart rhythms such as ventricular fibrillation.
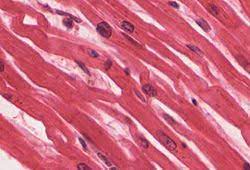
Cardiac muscle cells are roughly rectangular, measuring 100–150μm by 30–40μm.
Individual cardiac muscle cells are joined at their ends by intercalated discs to form long fibers.
Each cell contains myofibrils, specialized protein contractile fibers of actin and myosin that slide past each other.
The myofibrils are organized into sarcomeres, the fundamental contractile units of muscle cells.
The regular organization of myofibrils into sarcomeres gives cardiac muscle cells a striped or striated appearance when looked at through a microscope, similar to skeletal muscle.
These striations are caused by lighter I bands composed mainly of actin, and darker A bands composed mainly of myosin.
Cardiomyocytes contain T-tubules, pouches of cell membrane that run from the cell surface to the cell’s interior which improve the efficiency of contraction.
The majority of these cardiac muscle cells contain only one nucleus, unlike skeletal muscle cells which contain many nuclei.
Cardiac muscle cells contain many mitochondria which provide the energy needed for the cell in the form of adenosine triphosphate (ATP), making them highly resistant to fatigue.
T-tubules are microscopic tubes that run from the cell surface to deep within the cell.
T-tubules are continuous with the cell membrane, composed of the same phospholipid bilayer, and are open at the cell surface to the extracellular fluid that surrounds the cell.
T-tubules in cardiac muscle are larger than those in skeletal muscle, but fewer in number.
In the center of the cell they join the sarcoplasmic reticulum.
T-tubules rapidly transmitting electrical impulses (action potentials) from the cell surface to the cell’s core, and helping to regulate the concentration of calcium within the cell by excitation-contraction coupling, and are involved in mechano-electric feedback.
Intercalated discs are part of the cardiac muscle cell sarcolemma and they contain gap junctions and desmosomes.
The cardiac syncytium is a network of cardiomyocytes connected by intercalated discs.
The syncitium enables the rapid transmission of electrical impulses through the network, enabling the syncytium to act in a coordinated contraction of the myocardium.
An atrial syncytium and a ventricular syncytium are connected by cardiac connection fibers.
The electrical resistance of intercalated discs is very low, allowing free diffusion of ions.
It is the ease of ion movement along cardiac muscle fiber axes that allows the action potentials to travel from one cardiac muscle cell to the next, facing only slight resistance.
Each syncytium obeys the all or none law.
The intercalated discs connect the single cardiomyocytes to an electrochemical syncytium.
This is in contrast to the skeletal muscle, which becomes a multicellular syncytium during embryonic development.
The discs force transmission during muscle contraction.
Intercalated discs consist of three different types of junctions that allow action potentials to spread between cardiac cells by permitting the passage of ions between cells, producing depolarization of the heart muscle.
The three types of junction act together as a single area composita.
The intercalated discs run perpendicular to the direction of muscle fibers.
Cardiac fibroblasts are supporting cells within cardiac muscle.
Cardiac fibroblasts are unable to provide forceful contractions like cardiomyocytes.
They are largely responsible for creating and maintaining the extracellular matrix which surrounds the cardiomyocytes.
Fibroblasts play a crucial role in responding to injury: myocardial infarction.
Following injury, fibroblasts can become activated and turn into myofibroblasts.
Myofibroblast cells which exhibit behavior somewhere between a fibroblast, which generate extracellular matrix, and a smooth muscle cell with the ability to contract.
Fibroblasts tend to repair an injury by creating collagen while contracting to pull the edges of the injured area together.
Fibroblasts are smaller but more numerous than cardiomyocytes.
Several fibroblasts can be attached to a cardiomyocyte at once.
Fibroblasts attached to a cardiomyocyte they can influence the electrical currents passing across the muscle cell’s surface membrane.
Fbroblast roles include electrical insulation of the cardiac conduction system, and the ability to transform into other cell types including cardiomyocytes and adipocytes.
The extracellular matrix (ECM) surrounds the cardiomyocyte and fibroblasts, and is composed of proteins including collagen and elastin along with polysaccharides known as glycosaminoglycans.
The extracellular matrix substances give support and strength to the muscle cells, create elasticity in cardiac muscle, and keep the muscle cells hydrated by binding water molecules.
The matrix in immediate contact with the muscle cells is the basement membrane, mainly composed of type IV collagen and laminin.
Cardiomyocytes are linked to the basement membrane via specialised glycoproteins called integrins.
People are born with a set number of heart muscle cells, or cardiomyocytes, which increase in size as the heart grows larger during childhood development.
Cardiomyocytes are slowly turned over during aging, but less than 50% of the cardiomyocytes present at birth are replaced during a normal life span.
The growth of individual cardiomyocytes not only occurs during normal heart development, in response to extensive exercise, heart disease, or heart muscle injury such as after a myocardial infarction.
A healthy adult cardiomyocyte has a cylindrical shape that is approximately 100μm long and 10–25μm in diameter.
Cardiomyocyte hypertrophy occurs through sarcomerogenesis, the creation of new sarcomere units in the cell.
During heart volume overload, cardiomyocytes grow through eccentric hypertrophy.
The cardiomyocytes extend lengthwise but have the same diameter, resulting in ventricular dilation.
During heart pressure overload, cardiomyocytes grow through concentric hypertrophy.
The cardiomyocytes grow larger in diameter but have the same length, resulting in heart wall thickening.
The physiology of cardiac muscle shares many similarities with that of skeletal muscle.
The primary function of both muscle types is to contract, and in both cases, a contraction begins with a characteristic flow of ions across the cell membrane known as an action potential.
The cardiac action potential subsequently triggers muscle contraction by increasing the concentration of calcium within the cytosol.
The cardiac cycle is the performance of the
heart from the beginning of one heartbeat to the beginning of the next.
The cardiac cycle consists of two periods: one during which the heart muscle relaxes and refills with blood, called diastole, following a period of robust contraction and pumping of blood, dubbed systole.
After emptying, the heart immediately relaxes and expands to receive another influx of blood returning from the lungs and other systems of the body, before again contracting to pump blood to the lungs and those systems.
A normal performing heart must be fully expanded before it can efficiently pump again.
The rest phase is considered polarized, and the resting potential during this phase of the beat separates the ions such as sodium, potassium, and calcium.
Myocardial cells possess the property of automaticity or spontaneous depolarization, the direct result of a membrane which allows sodium ions to slowly enter the cell until the threshold is reached for depolarization.
Calcium ions follow and extend the depolarization.
When calcium stops moving inward, potassium ions move out slowly to produce repolarization.
The very slow repolarization of the cardiomyocyte membrane is responsible for the long refractory period.
The action potential in cardiac muscle comprises an inward flow of both sodium and calcium ions.
The flow of sodium ions is rapid but very short-lived, while the flow of calcium is sustained and gives the plateau phase characteristic of cardiac muscle action potentials.
The is a small flow of calcium through the L-type calcium channels , which triggers a much larger release of calcium from the sarcoplasmic reticulum:calcium-induced calcium release.
Cardiac muscle fibers require calcium to be present in the solution surrounding the cell to contract, while skeletal muscle fibers will contract without extracellular calcium.
