 An atom is the basic unit of matter.
An atom is the basic unit of matter.
It is the smallest particle of an element that retains the chemical properties of that element.
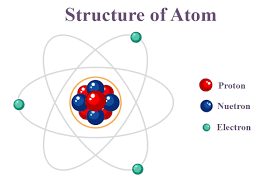
Atoms consist of a positively charged nucleus at the center, which is made up of protons and neutrons, surrounded by negatively charged electrons that orbit the nucleus.
Atoms consist of a small positively charged nucleus, made of positively charged protons and uncharged neutrons, surrounded by a cloud of negatively charged electrons; the charges cancel out, so atoms are neutral.
Electrons participate in chemical reactions, but the nucleus does not.
Each distinct atomic number therefore corresponds to a class of atom: these classes are called the chemical elements.
When atoms participate in chemical reactions, they either gain or lose electrons to form positively- or negatively-charged ions; or share electrons with each other.
The number of protons in an atom’s nucleus determines what element it is, while the number of neutrons and electrons can vary.
Atoms are the basic building blocks of all matter.
Atoms are extremely small, typically around 100 picometers across.
A human hair is about a million carbon atoms wide.
Atoms are the smallest units of matter that retain the properties and characteristics of a particular chemical element.
Each atom consists of a central nucleus containing protons and neutrons, which are surrounded by electrons that orbit the nucleus.
The number of protons in the nucleus of an atom determines the element it represents.
This number is called the atomic number and is represented by the symbol “Z”.
The total number of protons and neutrons in the nucleus is called the atomic mass number and is represented by the symbol “A”.
The electrons in an atom are arranged in energy shells or orbitals around the nucleus.
Each shell can hold a specific number of electrons, and the outermost shell is known as the valence shell.
The electrons in the valence shell determine the chemical behavior of the element, including its reactivity and ability to form chemical bonds with other atoms.
Atoms can combine with other atoms to form molecules or compounds, which play a vital role in chemical reactions and the formation of various substances.
Understanding the properties and behavior of atoms is essential inchemistry, physics, and materials science.
Atoms can be subdivided into different types based on the number of protons (and thus also electrons) they have.
This is called the atomic number, often symbolized Z.
For example, any atom that contains 11 protons is sodium, and any atom that contains 29 protons is copper.
The number of neutrons defines the isotope of the element.
It is the smallest recognized division of a chemical element.
Atoms cannot be seen with conventional microscopes.
Atoms are so small that accurately predicting their behavior using classical physics is not possible due to quantum effects.
More than 99.94% of an atom’s mass is in the nucleus.
The protons have a positive electric charge, the electrons have a negative electric charge, and the neutrons have no electric charge.
If the number of protons and electrons are equal, then the atom is electrically neutral.
If an atom has more or fewer electrons than protons, then it has an overall negative or positive charge, respectively – such atoms are called ions.
The electrons of an atom are attracted to the protons in an atomic nucleus by the electromagnetic force.
The protons and neutrons in the nucleus are attracted to each other by the nuclear force, which is stronger than the electromagnetic force that repels the positively charged protons from one another.
If the repelling electromagnetic force becomes stronger than the nuclear force, the nucleus splits and leaves behind different elements, a form of nuclear decay.
Atoms can attach to one or more other atoms by chemical bonds to form chemical compounds such as molecules or crystals.
Neutrons and protons were found to be hadrons, or composites of smaller particles called quarks.
The atom is composed of various subatomic particles.
The constituent particles of an atom are the electron, the proton and the neutron.
The electron is by far the least massive of these particles at 9.11×10−31 kg, with a negative electrical charge and a size that is too small to be measured using available techniques.
It was the lightest particle with a positive rest mass measured, until the discovery of neutrino mass.
Electrons are bound to the positively charged nucleus by the attraction created from opposite electric charges.
If an atom has more or fewer electrons than its atomic number, then it becomes respectively negatively or positively charged as a whole; a charged atom is called an ion.
Protons have a positive charge and a mass 1,836 times that of the electron, at 1.6726×10−27 kg.
The number of protons in an atom is called its atomic number.
Neutrons have no electrical charge and have a free mass of 1,839 times the mass of the electron, or 1.6749×10−27 kg.
Neutrons are the heaviest of the three constituent particles.
Neutron mass can be reduced by the nuclear binding energy.
Neutrons and protons, known as nucleons, have comparable dimensionson the order of 2.5×10−15 m—although the surface of these particles is not sharply defined.
Electrons are truly elementary particles with no internal structure, whereas protons and neutrons are composite particles composed of elementary particles called quarks.
There are two types of quarks in atoms, each having a fractional electric charge.
The quarks are held together by the strong force which is mediated by gluons.
The protons and neutrons, in turn, are held to each other in the nucleus by the nuclear force.
All the bound protons and neutrons in an atom make up a tiny atomic nucleus, and are collectively called nucleons.
The radius of a nucleus is approximately equal to 1.07 femtometres, much smaller than the radius of the atom, which is on the order of 105 fm.
Atoms of the same element have the same number of protons, called the atomic number.
Within a single element, the number of neutrons may vary, determining the isotope of that element.
The total number of protons and neutrons determine the nuclide.
The number of neutrons relative to the protons determines the stability of the nucleus, with certain isotopes undergoing radioactive decay.
The proton, the electron, and the neutron are classified as fermions.
Atoms with matching numbers of protons and neutrons are more stable against decay, but with increasing atomic number, the mutual repulsion of the protons requires an increasing proportion of neutrons to maintain the stability of the nucleus.
The fusion of two nuclei that create larger nuclei with lower atomic numbers than iron and nickel—a total nucleon number of about 60—is usually an exothermic process that releases more energy than is required to bring them together.
It is this energy-releasing process that makes nuclear fusion in stars a self-sustaining reaction.
For heavier nuclei, the binding energy per nucleon in the nucleus begins to decrease.
Fusion processes producing nuclei that have atomic numbers higher than about 26, and atomic masses higher than about 60, are endothermic processes.
The electrons in an atom are attracted to the protons in the nucleus by the electromagnetic force.
This force binds the electrons inside an electrostatic potential well surrounding the smaller nucleus, which means that an external source of energy is needed for the electron to escape.
Electrons bound near the center of the potential well require more energy to escape than those at greater separations.
Electrons have properties of both a particle and a wave.
The electron cloud is a region inside the potential well where each electron forms a type of three-dimensional standing wave.
It is wave form that does not move relative to the nucleus, and its behavior is defined by an atomic orbit.
A atomic orbit is mathematical function that characterizes the probability that an electron appears to be at a particular location when its position is measured.
Only a discrete set of these orbitals exist around the nucleus, as other possible wave patterns rapidly decay into a more stable form.
Orbitals can have one or more ring or node structures, and differ from each other in size, shape and orientation.
Each atomic orbital corresponds to a particular energy level of the electron.
The electron can change its state to a higher energy level by absorbing a photon to boost it into the new quantum state.
Through spontaneous emission, an electron in a higher energy state can drop to a lower energy state while radiating the excess energy as a photon.
Atoms are electrically neutral if they have an equal number of protons and electrons.
Atoms that have either a deficit or a surplus of electrons are called ions.
Electrons that are farthest from the nucleus may be transferred to other nearby atoms or shared between atoms.
This mechanism allows atoms to be able to bond into molecules and other types of chemical compounds like ionic and covalent network crystals.
Any two atoms with an identical number of protons in their nuclei belong to the same chemical element.
Atoms with equal numbers of protons but a different number of neutrons are different isotopes of the same element.
All known isotopes of elements with atomic numbers greater than 82 are radioactive.
About 339 nuclides occur naturally on Earth.
251, 74%, have not been observed to decay, and are referred to as stable isotopes.
35 radioactive nuclides have half-lives longer than 100 million years, and are long-lived enough to have been present since the birth of the Solar System:primordial nuclides.
53 short-lived nuclides are known to occur naturally, as daughter products of primordial nuclide decay, such as radium from uranium, or as products of natural energetic processes on Earth, such as cosmic ray bombardment.
For 80 of the chemical elements, at least one stable isotope exists.
There is only a handful of stable isotopes for each of these elements, the average being 3.1 stable isotopes per element.
The large majority of an atom’s mass comes from the protons and neutrons that make it up.
The total number of these particles (nucleons) is called the mass number.
The actual mass of an atom at rest is often expressed in daltons (Da), also called the unified atomic mass unit (u).
Hydrogen-1 (the lightest isotope of hydrogen which is also the nuclide with the lowest mass) has an atomic weight of 1.007825 Da.
One mole of atoms of any element always has the same number of atoms (about 6.022×1023).
If an element has an atomic mass of 1 u, a mole of atoms of that element has a mass close to one gram.
Each carbon-12 atom has an atomic mass of exactly 12 Da, and so a mole of carbon-12 atoms weighs exactly 0.012 kg.
Theatomic radius is a measure of the distance out to which the electron cloud extends from the nucleus.
Atomic radii may be derived from the distances between two nuclei when the two atoms are joined in a chemical bond.
The atom radius varies with the location of an atom on the atomic chart, the type of chemical bond, the number of neighboring atoms and a quantum mechanical property known as spin.
The smallest atom is helium with a radius of 32 pm, while one of the largest is caesium at 225 pm.
When subjected to external forces, like electrical fields, the atom’s shape may deviate from spherical symmetry.
Atomic dimensions are thousands of times smaller than the wavelengths of light (400–700 nm) so they cannot be viewed using an optical microscope.
A typical human hair is about 1 million carbon atoms in width.
A single drop of water contains about 2 sextillion (2×1021) atoms of oxygen, and twice the number of hydrogen atoms.
If an apple were magnified to the size of the Earth, then the atoms in the apple would be approximately the size of the original apple.
Every element has one or more isotopes that have unstable nuclei that are subject to radioactive decay, causing the nucleus to emit particles or electromagnetic radiation.
The most common forms of radioactive decay are:
Alpha decay: in this process nucleus emits an alpha particle, which is a helium nucleus consisting of two protons and two neutrons.
The result of the emission is a new element with a lower atomic number.
Beta decay is regulated by the weak force, and result from a transformation of a neutron into a proton, or a proton into a neutron.
The electron or positron emissions are called beta particles.
Beta decay either increases or decreases the atomic number of the nucleus by one.
Electron capture is more common than positron emission, because it requires less energy.
With electron capture type of decay, an electron is absorbed by the nucleus, rather than a positron emitted from the nucleus.
A neutrino is still emitted in this process, and a proton changes to a neutron.
Gamma decay: this process results from a change in the energy level of the nucleus to a lower state, resulting in the emission of electromagnetic radiation.
The excited state of a nucleus which results in gamma emission usually occurs following the emission of an alpha or a beta particle.
Thus, gamma decay usually follows alpha or beta decay.
Rare types of radioactive decay include ejection of neutrons or protons or clusters of nucleons from a nucleus, or more than one beta particle.
Each radioactive isotope has a characteristic decay time period—the half life—that is determined by the amount of time needed for half of a sample to decay.
This is an exponential decay process that steadily decreases the proportion of the remaining isotope by 50% every half-life.
The chemical elements are often displayed in a periodic table that is laid out to display recurring chemical properties, and elements with the same number of valence electrons form a group that is aligned in the same column of the table.
Ubiquitousness and stability of atoms relies on their binding energy.
An atom has a lower energy than an unbound system of the nucleus and electrons.
Isotopes such as lithium-6, as well as some beryllium and boron are generated in space through cosmic ray spallation, and occurs when a high-energy proton strikes an atomic nucleus, causing large numbers of nucleons to be ejected.
Carbon-14 is continuously generated by cosmic rays in the atmosphere.
Some atoms on Earth have been artificially generated either deliberately or as by-products of nuclear reactors or explosions.
The Earth contains approximately 1.33×1050 atoms.
99% of the atmosphere is bound in the form of molecules, including carbon dioxide and diatomic oxygen and nitrogen.
At the surface of the Earth, an overwhelming majority of atoms combine to form various compounds, including water, salt, silicates and oxides.
Atoms can also combine to create materials that do not consist of discrete molecules, including crystals and liquid or solid metals.
All nuclides with atomic numbers higher than 82 are known to be radioactive.
No nuclide with an atomic number exceeding 92 (uranium) exists on Earth as a primordial nuclide, and heavier elements generally have shorter half-lives.
Predictions for the half-life of the most stable nuclide on the island range from a few minutes to millions of years.
Each particle of matter has a corresponding antimatter particle with the opposite electrical charge.
